Editor: 邵丹蕾 Author: Sun et al. Time: 2018-07-11 Number of visits :346
From Atmospheric chemistry group
Sun et al., JGR-A. 2018 doi:10.1002/2017JD027264
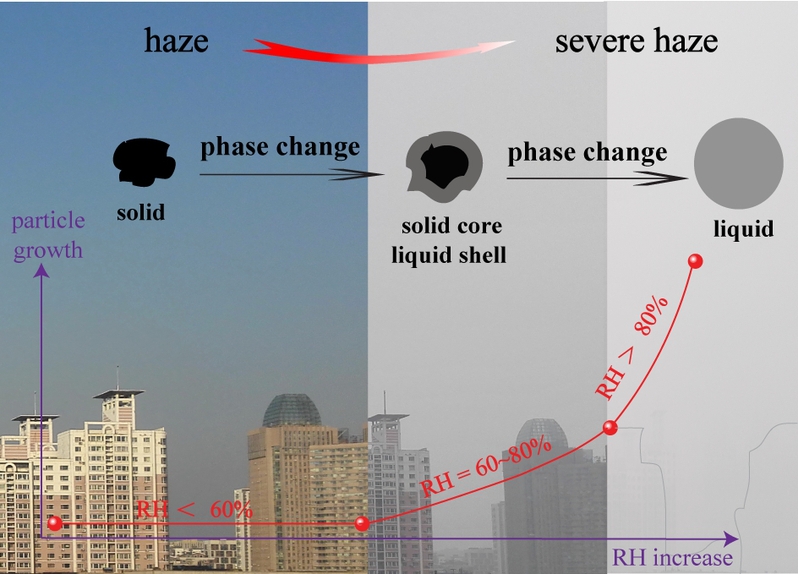
Recently, aerosol water has received more attention because heterogeneous reactions of SO2, NO2, and NH3 on wet particles accelerate the severe haze formation in North China. Ammonium sulfate and ammonium nitrate (AS-AN) are key components of fine urban particles. Especially, nitrate concentration keeps increasing in polluted air in China. Our study indicates the increase of AN content promotes the occurrence of aqueous shell at lower RH. Here we found that most of urban particles generally keep solid core and aqueous shell at RH = 60~80% and aqueous phase at RH>80%. These findings can clearly explain the role of nitrate in phase transitions and make up the discussion about heterogeneous reactions on particle surfaces during the severe hazes in North China. Humidity-dependent phase states of particles are useful for interpreting the secondary aerosols’ formation in severe hazes as well as in modeling studies.
Reference: Sun, J., Liu, L., Xu, L., Wang, Y., Wu, Z., Hu, M., … Li, W. (2018). Key role of nitrate in phase transitions of urban particles: Implications of important reactive surfaces for secondary aerosol formation. Journal of Geophysical Research: Atmospheres, 123, 1234–1243. https://doi.org/10.1002/2017JD027264
